JSS College of Pharmacy, Mysore
Webinar on Medical Device Rules 2017 and Software as a Medical Device
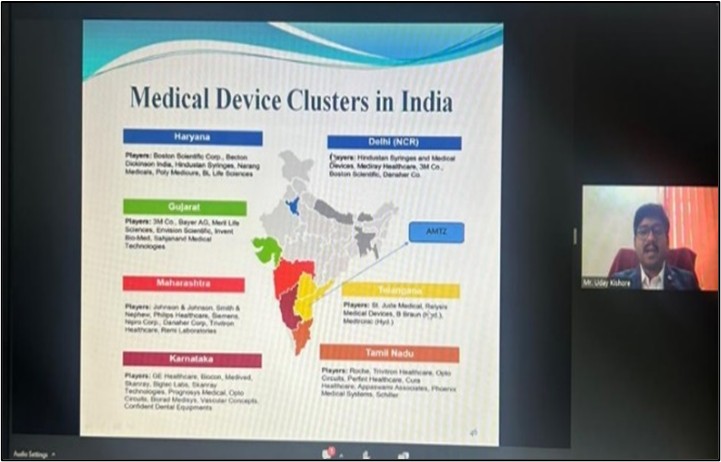
The session commenced with Mr. Uday Kishore providing a concise overview of the Drug and Cosmetics Act in India. He proceeded to discuss the regulations pertaining to Medical Devices, the general regulatory landscape for medical devices, classification of medical devices, the potential for the medical device industry, and the mandatory regulatory requirements that must be met by medical device manufacturers seeking market authorization approval in India. Additionally, he briefly highlighted educational institutions that offer courses related to medical devices.
Read More